Each year thousands of patients undergo hip replacement and knee replacement surgery. Unfortunately, several hip implant and knee implant devices used have been recalled due to unsafe product defects and adverse side effects.
The Food and Drug Administration (FDA) became aware of the risks associated with metal hip implants in 2011 ordering all manufacturers to conduct independent studies into how often these devices were failing. This resulted in multiple recalls, but not until after thousands were suffering from severe adverse effects and even having to undergo revision surgery.
Additionally, more recalls have happened in the years since, not only encompassing metal hip replacements, but also includes recalls of the individual parts of other kinds of hip and knee replacements which have led to pain and injury for those affected.
If a defective product has injured you or a loved one, contact a North Carolina metal hip implants lawyer at Daggett Shuler injury & disability lawyers to protect your legal rights. We want to help you hold these thoughtless or careless manufacturers accountable for the unsafe products they market to the unsuspecting public.
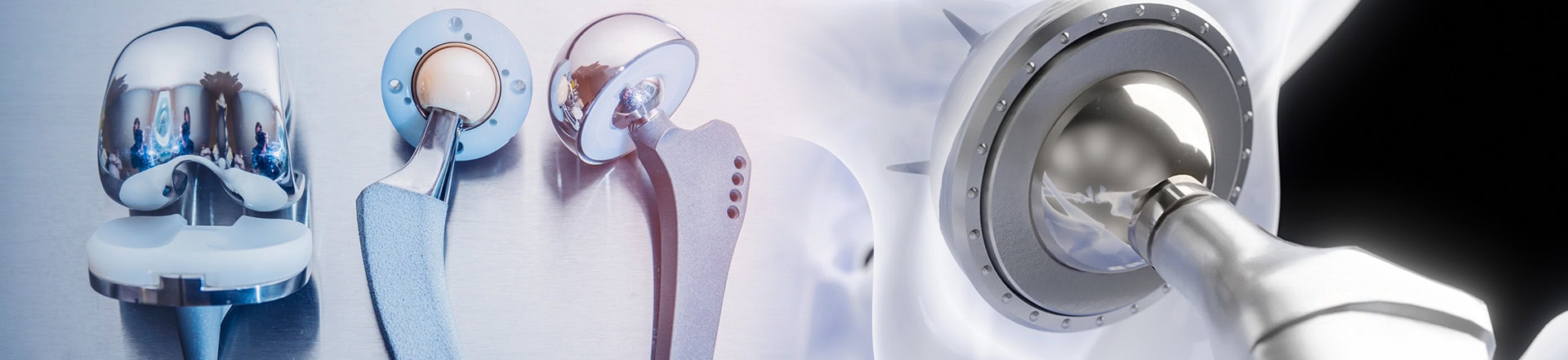
DePuy Orthopedics, a division of Johnson & Johnson, had multiple hip replacements recalled by the Food and Drug Administration. Prior to the recall approximately 93,000 patients around the world received a DePuy ASR hip implant. The following devices manufactured by Johnson & Johnson were recalled:
In 2010 the FDA removed both devices from the market due to the high failure rate and severe side effects associated with the devices.
The recalled devices are constructed using metal components that rub together and corrode during normal use, causing flakes of metal to enter the blood stream, bone and surrounding tissues. The corrosion left patients with severe side effects including:
In 2012 the Stryker Rejuvenate was recalled due to the serious damage and health risks associated with the hip replacement product.
Once the Zimmer products actually started to be used by patients, it became clear that there were serious problems associated with the devices as well, independent researchers confirmed these problems after the product was released.
In multiple cases, when patients experience these complications, at least one revision surgery is required. The patient has to deal with the pain of the complications and potentially undergo a painful and expensive surgery due to the defective product.
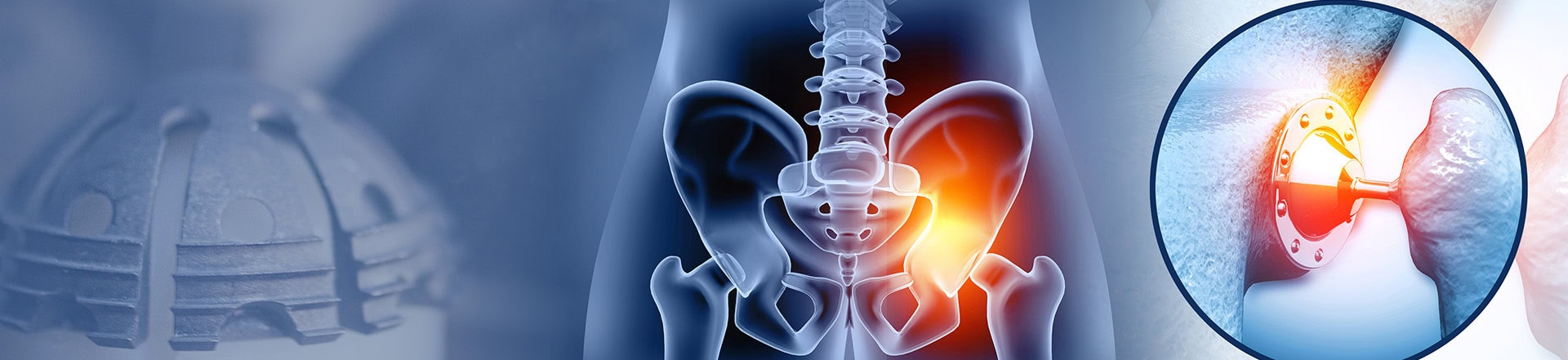
The Stryker Corporation, one of the leading manufacturers for medical devices, including hip implant devices, has had numerous safety alerts issued by the Food and Drug Administration on multiple Stryker products. The LFit® V40, one of Stryker Corporation’s products, has received a high volume of complaints issued by doctors and patients due to the femoral head components. The devices are prone to high failure rates and defects.
When the metal hip implant devices fail patients suffer from serious complications and health risks. Patients may have to undergo revision surgery, that is not only painful but costly, to remove the defective device.
In 2012 The Food and Drug Administration (FDA) announced that the Stryker Corporation issued a voluntary recall of their Rejuvenate and ABG ll metal hip implant devices. After repeated testing, it was proven that the artificial metal joints in the devices would corrode and break down quickly due to normal wear and tear from the body.
Symptoms of failure and break down of the LFit V40 include:
The corrosion then causes increasing amounts of toxic metal debris to enter a patient’s surrounding tissue and bloodstream leading to multiple dangerous side effects.
Cobalt or chromium poisoning
High levels of metal in the bloodstream, a condition known as metallosis
Pain in the groin, hip or leg
Painful and pronounced limp
Swelling on and around the hip joint
Revision surgery


Our attorneys have helped clients with legal support through workers’ compensation, Social Security Disability, personal injury, car accident claims, and more. Our clients know that we stand beside them when they are going through a difficult time. Read reviews from real clients, and if you had a positive experience with Daggett Shuler Law, please consider leaving a review.
Contact the attorneys at Daggett Shuler today for a free, no-obligation consultation. You can depend on us.